Introduction
To be successful in the business world, there is a given: in addition to mastering one’s trade, whatever it may be, one must also master all the other supporting aspects, administration, human resources, inventory management, communication, etc.
If one, just one, of these parts is left behind, in the best-case scenario business will be slower than what it could be. In the worst-case scenario, the company is heading towards dire straits eyes wide shut.
In this context a laboratory that wins is a laboratory that does not waste time managing and supervising complex processes and/or correcting errors and oversights. It turns all of it over to well-built and well-maintained systems.
For testing, sampling and calibration laboratories (cf. ISO 17025), the combination of scientific, administrative and commercial processes makes these tasks complex and the need to use an information system that takes into account the entirety and interlaces the different elements is paramount. This is called an integrated system or an Enterprise Resource Planning (ERP).
What is a LIMS?
LIMS stands for Laboratory Information Management System. It is a term that covers a lot of things because laboratories are of different types and specialties. There are more 150 LIMS on the market, with a handful holding on to the lion’s share.
A LIMS comprises 3 main parts:
- The laboratory that manages the registration of materials or measurement, which will be the subject of analysis requests, analyses and reports. Not to forget samples, reagents, laboratory devices (calibrations, maintenance, etc.).
- The commercial part with orders and payments
- The administrative part with the general management, accounting, HR etc.
In the last 30 years, with the emergence of IT, laboratories have started to use existing tools to simplify operations, resulting in a pile of separate applications, “more or less” connected to each other. The problems of side-effects are obvious and finally the expected gain is difficult to assess. So, this is one problem but unfortunately neither the only nor the most important one.
The biggest concern is the lack of respect for scientific laboratory processes which prevents analysis integrity not to mention the incapacity to “demonstrate that they operate competently and generate valid results, thereby promoting confidence in their work both nationally and around the world” (cf. ISO 17025).
Thus, it is not uncommon to note the absence of a scientific process in the gathering and recording of results in both commercial and academic laboratories as well as the unhealthy interweaving of administrative and scientific matters.
The laboratory is one thing, administration is another.
An elementary example is when, on the order form, the system allows users to enter “presupposed” scientific data when the analysis request has not even been validated nor the sample generated.
Basic process of analysis configuration
Before being put into production in the laboratory, the LIMS must be configured. Ideally, the user should be able to do this autonomously and without the intervention of the software publisher.
The objective is to define the parameter characteristics that will be used for a given analysis., i.e., by that we mean the variable elements that will be used to describe a given phenomenon.
The parameter characteristic is therefore the assembling of a variable number of factors, as per the diagram below:
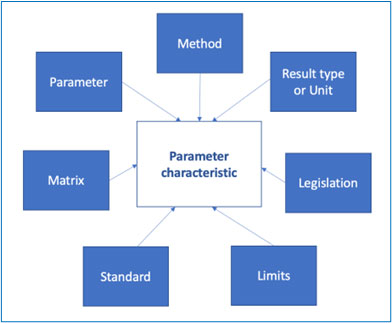
One could also link to a given standard, the reference of the document that describes the method and other information related to the method itself etc.
It should be noted that not all laboratories are obligated to such precise standards. In certain sectors, the constraints are much less, and the intrinsic standards, norms and legislation non-existent.
Basic administrative, commercial and laboratory process
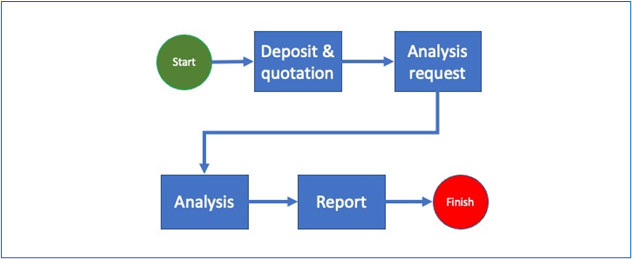
The basic process of any analysis or calibration begins with the deposit of materials or data. At this stage the laboratory issues a purchase order for one or more given services and a deposit slip which must describe precisely what has been deposited as well as (if applicable) the physical and chemical conditions (ex.: temperature, hygrometry etc.).
Following this, the analysis request is registered in the system and the samples are generated. Note that even in the case of non-destructive analysis, the deposited material is different from the sample because the sample refers to a specific material at a specific time in specific conditions.
The analysis request is then processed, firstly to validate the relevance of the service ordered in view of the material deposited and secondly to trigger the sending of the generated sample to the laboratory and possibly specify other information such as priority, department/laboratory technician(s) in charge etc.
The sample is now in the laboratory and undergoes one or a series of tests that will be encoded, validated (once or more times) and allow the generation of the final report which will be signed by the person in charge.
If the tests are inconclusive and require a “Rework”, the system should request for the reasons and record the answers.
Conclusion
This is a very basic LIMS process for the writing of this article. But it serves its purpose.
Using a LIMS in a laboratory is fundamental. It simplifies the workflow, makes the daily operations smoother and ensure a high level of security. The system must record every interaction: who, when, where, what. Every element must be referenced separately, whether a deposit, a quote, a sample, an analysis request, an analysis, a report etc.
The system should also be positioned on monitored, load-balanced servers that ensure continuity in case of problems and connection to the latter should implement the highest level of security.
Last but not least: implementing a LIMS is not just buying a license, it’s a full-fledged project. If you are thinking of moving to a LIMS, make sure the company you are choosing to work with has the profile to manage the implementation, configuration and training/support that goes with it.
A LIMS should have the capacity to retrieve data directly from the devices that will be exploited allowing for another level of results. And if you If you choose to work with Odoo, SAP or Oracle this allows to attach specific modules to the LIMS, such learning management systems (LMS) or research and project management infrastructure, logistics, maintenance, Q&A etc.
Using methodologies such as Agile and Scrum are lynchpins to successful projects.












